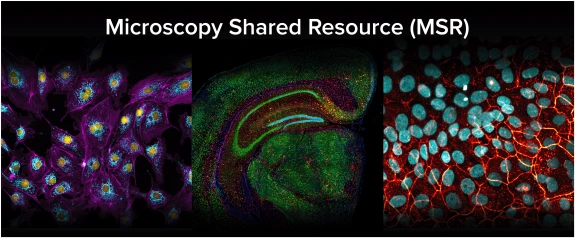
The Microscopy Shared Resource (MSR) is a new, advanced microscopy facility located at the University of Arizona Cancer Center, servicing the immediate needs and future demands of biomedical and translational researchers. The MSR provides several advanced instruments and services for all university researchers, including confocal microscopy, super-resolution microscopy, slide scanning, live cell imaging, multiphoton and intravital imaging, advanced image analysis, and much more. In addition to providing advanced instrumentation and imaging services, the core facility is a model of academic collaboration. MSR provides a space for on-site workshops, imaging seminars highlighting cutting-edge imaging approaches, demonstrations of next components/instruments, and support to the broader imaging community by building an “imaging culture” on campus.
Download the MSR Information Booklet
MSR Imaging Services and Instruments
The MSR has 6 advanced Nikon microscopes with Nikon NIS Elements image acquisition and analysis software, allowing for continuity between instruments and a shared hardware and software experience for all users.
The following are services offered at the MSR:
⦁ Nikon AX R Laser-Scanning Confocal System
⦁ Nikon Crest Spinning-Disk Confocal System
⦁ Nikon SoRa Spinning-Disk Confocal System
⦁ Nikon STORM Super Resolution System*
⦁ Nikon AX R Multiphoton System*
*Due to the advanced nature of this imaging method, this instrument is generally assisted-use only. However, training is available for advanced users that plan to regularly use this instrument.
For training on any of these instruments, please contact the MSR Manager, Marco Padilla (marco@arizona.edu).
⦁ Imaging Consultation/Experimental Design: Initial meeting (~30min) with all potential users and MSR staff to discuss imaging and experimental needs. From this meeting, users will be recommended the appropriate microscope and schedule training for the appropriate microscope.
⦁ Microscope Training: Instrument training is generally 2 – 4hrs, split into 2 training sessions. Training #1 focuses on the basics in operating the microscope with an MSR-provided sample and Training #2 focuses on building an imaging workflow based on user needs using a user-provided sample. Required training time is adjusted based on the user’s familiarity with the imaging technique and Nikon hardware and software. Users can be trained on multiple MSR instruments.
⦁ Slide Scanning Service: MSR will perform brightfield and fluorescent imaging on user provided samples using the Nikon BioPipeline High-Content System.
⦁ Assisted STORM Imaging Service: MSR staff provides assisted STORM imaging for users with no prior experience.
⦁ Assisted Multiphoton Imaging Service
Image Analysis Training: MSR staff trains users on how to use image analysis platforms to build their own analysis pipeline. Recommended for users that are inclined to learn how to build image analysis tools for current and future projects. Currently, MSR is training users in ImageJ/Fiji, QuPath, and Nikon NIS Elements software platforms.
Image Analysis Development: MSR staff develops an image analysis script for users to perform semi- to fully automated image analysis. MSR staff meets with users to design a custom analysis script based on their quantification needs. Recommended for users that need assistance developing an analysis tool but will carry out the analysis for their project. Currently, the MSR develops analysis scripts for users in QuPath and Nikon NIS Elements using General Analysis 3 (GA3).
- Image Analysis Full Service: MSR staff performs all steps of the quantification process - analysis development, image analysis on user-provided dataset, data export, and manuscript methods for analysis. Depending on the complexity of the analysis, script development will be performed in either QuPath or Nikon NIS Elements.

The Nikon "Crest" system is an inverted, spinning-disk confocal microscope that allows for high-throughput, confocal imaging of cell and tissue samples. The Crest system is equipped with a stage-top environmental incubator for live cell imaging and a back-illuminated sCMOS camera, ideal for imaging living samples (cells, organoids, etc.) with minimal phototoxicity.

The Nikon “AX R” system is an inverted laser-scanning confocal microscope and is our most capable instrument. The AX R has both a traditional, high-resolution Galvano scanner and a high-speed Resonant scanner with up to 2K X 2K resolution, making this instrument capable of both high-resolution and high-speed imaging.

The Nikon “SoRa” system is our most advanced spinning-disk confocal and merges the advantages of confocal imaging with a 2x increase in resolution using “SoRa mode”, making this both a confocal and super-resolution microscope.

The Nikon “Storm” system is our super-resolution microscope, capable of achieving a 10x increase in resolution than a wide-field fluorescence microscope
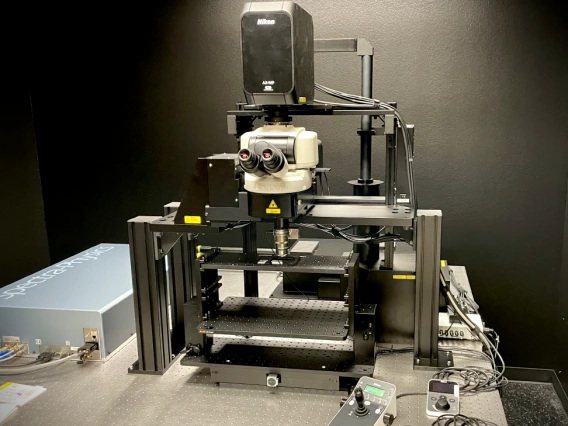
The Nikon “AX R MP” microscope is an upright multiphoton system ideal for intravital imaging and thick samples. This instrument is equipped with dual IR lasers for fast multichannel imaging, the same highspeed Resonant scanner in our AX R system, and a 22mm field of view, allowing more data to be collected in a single image. Due to the nature of this advanced technique, the AX R MP system is assisted-use only and the MSR provides a bench area for setup and ancillary equipment.

The Nikon “BioPipeline” is an automated, widefield slide scanning system equipped with a Marzhauser Slide Express 2 loader with a 120-slide capacity for high-throughput imaging, optics for polarized microscopy, and optics to support UV – IR imaging. Due to the robotic slide loader, the BioPipeline is ideal for high content projects that require imaging several slides in an automated fashion and supports both 1x3 and 2x3 glass slides. The BioPipeline is an assisted-use only system and is offered as a service at the MSR.
MSR Image Analysis Stations
The MSR Image Analysis Workstation is a dedicated analysis computer offered
to users conducting analysis of imaging datasets. While this workstation provides a streamlined workflow when acquiring images on Nikon microscopes offered within the MSR.
1. Can I bring live, biological samples to the MSR? Are there incubators or biosafety cabinets available for use?
a. Yes. The MSR supports live samples (up to BSL2 level) and users have access to 70% ETOH, paper towels, and nitrile gloves in each microscope room. Furthermore, all trained MSR users will have access to the MSR laboratory (UACC 0979) which includes a cell culture room with CO2 incubators, 37° bead bath, 4° fridge and -20° freezer, and general lab items (centrifuges, vortexes, micropipettes, etc.)
2. Do I need previous microscope experience to use the microscopes available at the MSR? Are there any training materials that I need before training?
a. No. Users are not required to have any background in the imaging method being utilized on the microscopes. Microscope training meets users where they are and is designed to make users fully independent.
3. I have used a confocal microscope before, do I still need training?
a. Yes. All new users to the MSR will undergo instrument training regardless of previous experience. This is to ensure that all users have been trained the same way before using MSR microscopes. However, the training scales to a user’s experience level and users with prior experience will be trained in less time.
4. What sample types (slides, dishes, chamber slides, multiwell plates, etc.) are compatible on MSR microscopes?
a. Supported sample types for each instrument vary, however, the details for each instrument (including supported samples) can be found in the “Learn More” section for each microscope.
5. Is there night and weekend access for MSR users?
a. Yes. All trained users have 24/7 building and room access and can schedule time on MSR confocal microscopes at any time. Building and room access will be made available during the training process.
6. Are there different microscope rates at different times of the day/week?
a. Yes. There are two different rates for trained users when using any of our 3 confocal microscopes – Unassisted and Overnight Imaging. The Unassisted rate ($35/hr) is from 8am – 6pm and the Overnight Imaging ($10/hr) is from 6pm – 8am, Monday – Sunday (no weekend rate). This rate change happens automatically in iLabs and does not need to be specified by the user when making the reservation. For full dvetails regarding our rates for each instrument, please visit our iLabs page and log in using your UA credentials (https://ua.ilab.agilent.com/service_center/6084/?tab=about).





